The depletion of the ozone layer
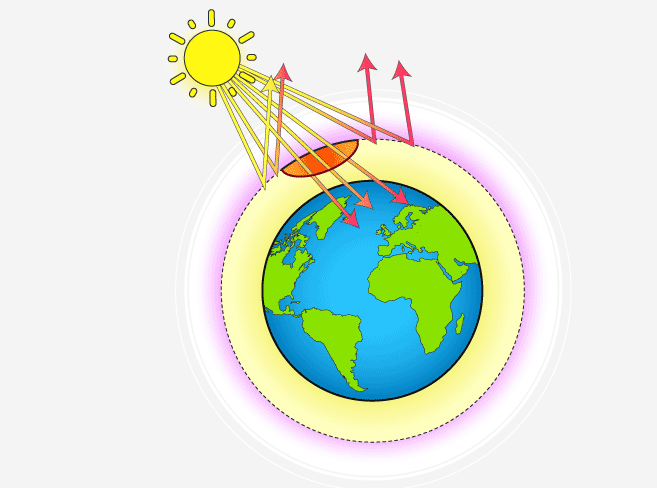
The depletion of the ozone layer, particularly in the stratosphere, is primarily caused by human activities that release certain chemicals known as ozone-depleting substances (ODSs) into the atmosphere. Here are the main causes of ozone layer depletion:
- Halons:
- Halons are similar to CFCs and contain bromine atoms in addition to chlorine and fluorine. They were commonly used in fire extinguishers and firefighting equipment.
- Like CFCs, halons release bromine and chlorine atoms when they break down in the stratosphere, contributing to ozone depletion through similar chemical reactions.
- Carbon Tetrachloride and Methyl Chloroform:
- These chemicals were used as solvents in industrial processes, particularly in the production of chemicals and electronics.
- They release chlorine atoms when they break down in the stratosphere, contributing to ozone depletion.
- Methyl Bromide:
- Methyl bromide is an agricultural fumigant used to control pests in soil and stored products.
- When released into the atmosphere, methyl bromide releases bromine atoms, which can contribute to ozone depletion.
- Nitrous Oxide (N2O):
- Nitrous oxide is a greenhouse gas and air pollutant emitted from agricultural activities, industrial processes, and combustion of fossil fuels.
- In the stratosphere, nitrous oxide can react with ozone, contributing to ozone depletion indirectly.
- Nitrogen Oxides (NOx):
- NOx compounds are released from combustion processes, such as those in vehicles, power plants, and industrial facilities.
- While primarily responsible for the formation of ground-level ozone (a component of smog), nitrogen oxides can also contribute to ozone depletion in the stratosphere through complex chemical reactions involving other ozone-depleting substances.
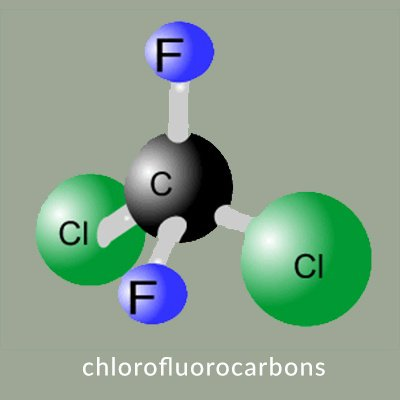
- CFCs are synthetic compounds containing chlorine, fluorine, and carbon atoms. They were widely used as refrigerants, solvents, propellants in aerosol sprays, and in the production of foam insulation.
- When released into the atmosphere, CFCs eventually reach the stratosphere, where they are broken down by ultraviolet (UV) radiation from the sun.
- The chlorine atoms liberated from CFCs react with ozone (O3) molecules, breaking them apart and reducing the ozone concentration in the stratosphere.