Understanding Protein Folding: Various Hypothesis
Protein folding is a complex process whereby a linear chain of amino acids, synthesized by the ribosome according to instructions encoded in the DNA, spontaneously folds into a three-dimensional structure with a specific shape and function. The process of protein folding is governed by various hypotheses and principles, each offering insights into the mechanisms underlying protein folding dynamics. Here, we’ll discuss some of the key hypotheses of protein folding:
- Anfinsen’s Thermodynamic Hypothesis:
- Proposed by Christian Anfinsen in 1973, this hypothesis states that the native structure of a protein is determined by its amino acid sequence and the surrounding environment.
- According to this hypothesis, proteins fold into their native conformation to minimize the free energy of the system, adopting the most stable configuration under physiological conditions.
- Anfinsen’s experiments with ribonuclease demonstrated that denatured proteins could refold into their native state when the denaturing agent was removed, supporting the idea that the primary sequence contains all the information necessary for folding.
- Levinthal’s Paradox:
- Proposed by Cyrus Levinthal in 1969, this paradox highlights the apparent contradiction between the vast number of possible conformations that a protein could explore during folding and the observed speed of folding.
- Given the large number of degrees of freedom in a polypeptide chain, it was initially thought that proteins would require an impractically long time to search all possible conformations before reaching their native state.
- Levinthal’s paradox suggests that proteins must follow a highly organized, non-random pathway to reach their native conformation efficiently, rather than exploring all possible conformations.
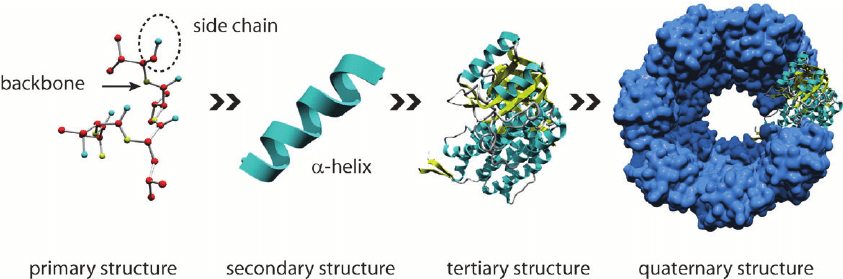
- The Hierarchical Model:
- The Energy Landscape Theory:
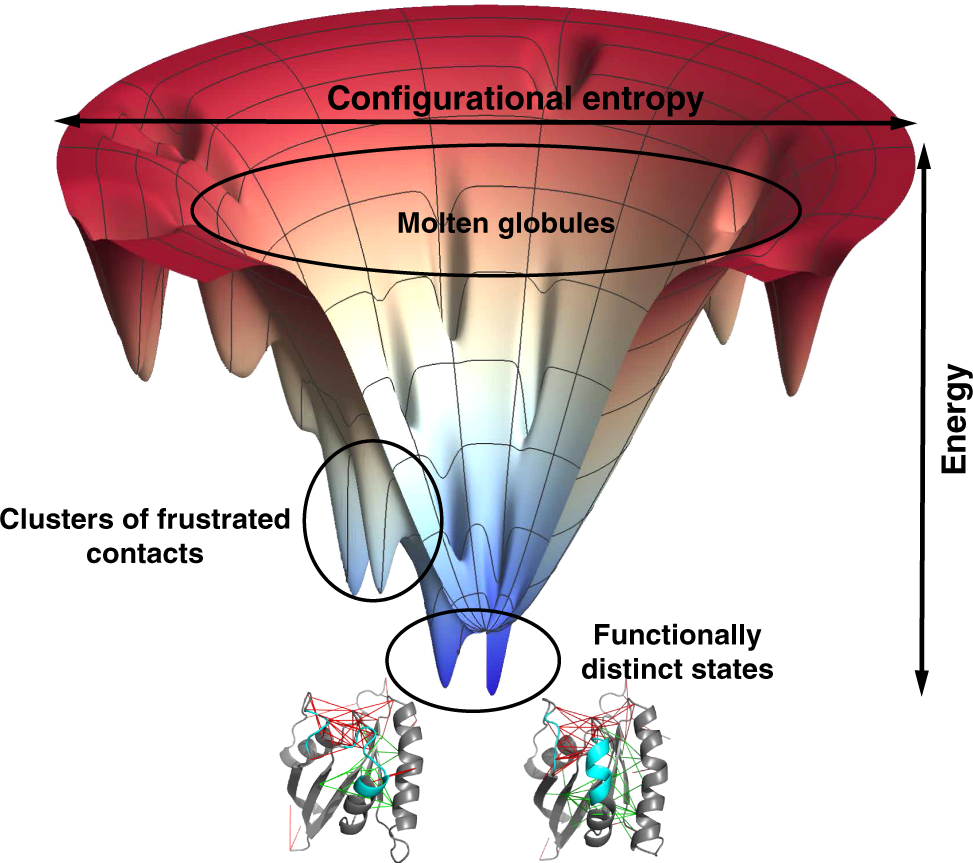
- This theory views protein folding as a process of navigating a complex energy landscape, where the protein must overcome energy barriers to reach its native state.
- Folding pathways are influenced by the shape of the energy landscape, which is determined by the interactions between amino acids and the surrounding environment.
- The energy landscape theory suggests that proteins can fold through multiple pathways, exploring various intermediate states before reaching their native conformation.
- The Foldon Model:
- Proposed by Peter Wolynes and co-workers, the foldon model posits that proteins contain modular folding units, known as foldons, which fold independently before assembling into the final three-dimensional structure.
- According to this model, proteins fold through a series of hierarchical folding events involving the cooperative formation of foldon units, which then come together to form the native structure.
- Foldons represent structurally independent units within the protein that fold autonomously, facilitating the rapid and efficient folding of complex protein structures.
- This model proposes that protein folding occurs in a hierarchical manner, with local interactions between adjacent amino acids driving the formation of secondary structure elements (e.g., α-helices and β-sheets), which then assemble into the tertiary structure of the protein.
- Secondary structure elements form through hydrogen bonding between nearby amino acids, stabilizing regular patterns of folding such as α-helices and β-sheets.
- Tertiary structure is determined by the packing of secondary structure elements and the interactions between distant regions of the polypeptide chain, including hydrophobic interactions, electrostatic forces, and disulfide bonds.
These hypotheses provide valuable insights into the mechanisms underlying protein folding and have guided experimental and computational studies aimed at elucidating the folding process. While each hypothesis offers a different perspective on protein folding dynamics, they collectively contribute to our understanding of how proteins achieve their functional native structures. Ongoing research continues to refine and expand upon these theories, shedding light on the fundamental principles governing protein folding.